Odoo For Morocco Pharmaceutical Industry
A connected Odoo ERP system that strengthens compliance, enhances production visibility, and streamlines supply chain coordination for Morocco’s pharma sector.
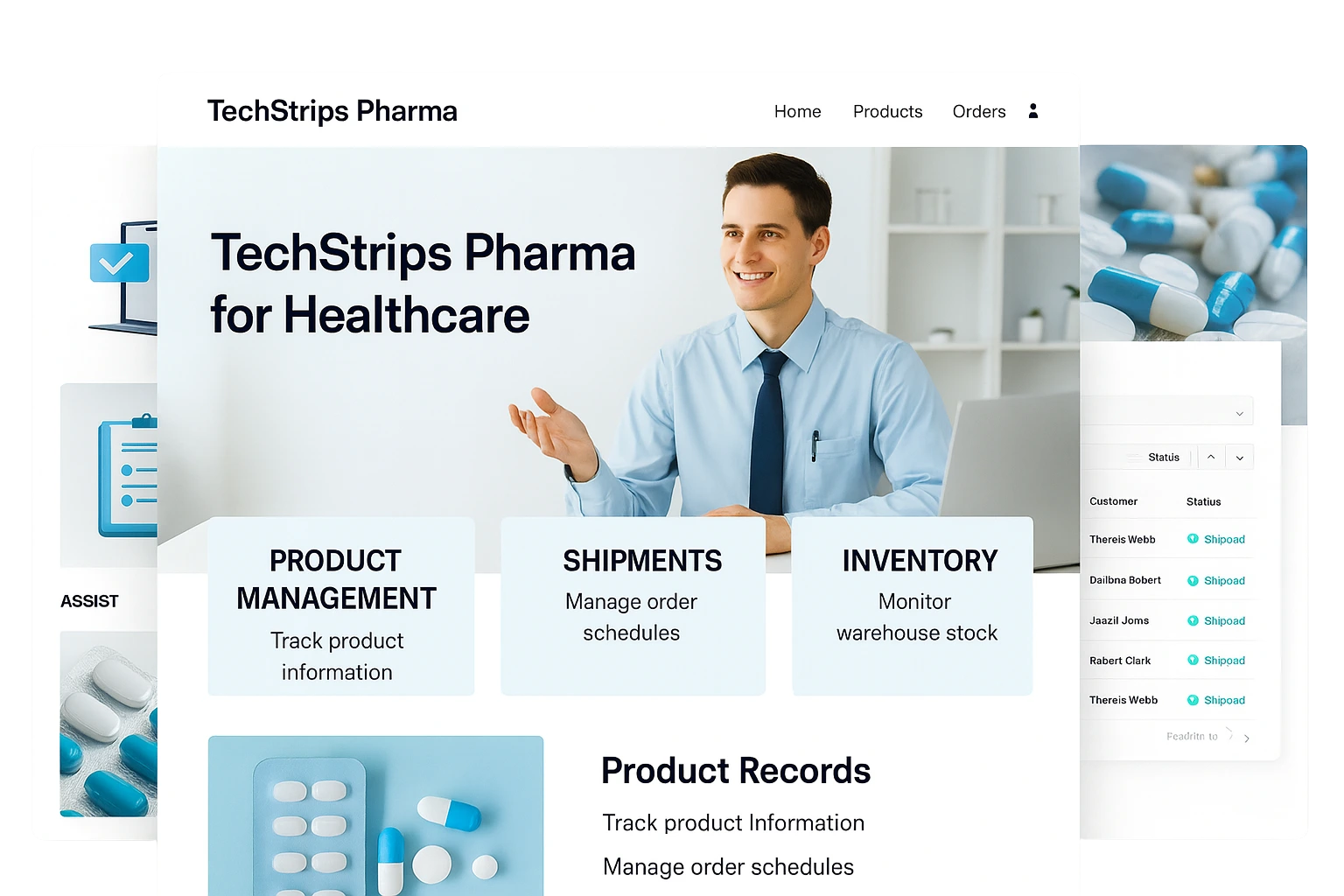
About
Morocco’s pharmaceutical industry operates under strict regulations requiring precise batch tracking, quality documentation, and end-to-end traceability. Many manufacturers still rely on disconnected tools, increasing compliance risks, production delays, and inventory inaccuracies. SDLC Corp implemented a unified Odoo ERP ecosystem that integrates manufacturing, quality control, inventory, procurement, finance, and distribution. With automated workflows and real-time dashboards, pharmaceutical companies gain accurate traceability, improved operational efficiency, and stronger regulatory compliance.
Industry
Pharmaceutical Manufacturing
Services
Odoo ERP, Compliance Automation, Batch & Inventory Management
Clients
Pharma Manufacturers, Distributors, R&D Facilities
Build your idea
How We Helped Morocco’s Pharma Sector Modernize with Odoo
Pharmaceutical companies in Morocco faced major challenges including manual compliance logs, limited visibility into production stages, and fragmented data across departments. These inefficiencies created delays, increased operational risks, and reduced accuracy in meeting regulatory standards. Inventory mismatches, manual QA processes, and isolated systems slowed both compliance and batch release cycles. We deployed a connected Odoo ERP environment covering manufacturing, QA, inventory, procurement, HR, and finance. Batch management, quality checks, and material consumption became automated and fully traceable.
Supply chain modules improved coordination between production, warehousing, and distribution. Automated compliance documentation simplified audits, while finance and HR modules improved cost control, scheduling, and regulatory reporting. The unified system enabled pharmaceutical firms to achieve real-time traceability, higher production efficiency, and faster decision-making across all operational layers,

“Our mission was to build a unified Odoo platform capable of managing large-scale projects across Saudi Arabia with complete operational clarity. By bringing procurement, timelines, material tracking, and financial oversight into one ecosystem, we empowered construction companies to work faster, reduce bottlenecks, and achieve complete cost transparency.”
Kishan Srivastava
CEO
Our Challenges
1
Manual Compliance Tracking
High risk of documentation errors, delayed approvals, and audit failures.
2
Limited Supply Chain Visibility
Automated purchase requests, vendor approvals, and material movement.
3
Inefficient Production Planning
Uncoordinated schedules causing delays, bottlenecks, and material waste.
4
Fragmented Operational Systems
Disconnected tools across R&D, manufacturing, QA, warehousing, and logistics.
5
Inventory & Batch Inaccuracies
Mismatches in raw materials, batch counts, expiry tracking, and finished products.
6
Quality Control Limitations
Manual QA checks lacking traceability, standardization, and automated workflows.
Transform Your Pharma Operations in Morocco into a $14M Compliance Success!

Our Solutions
1
Unified Odoo Real Estate ERP
One connected system for manufacturing, QA, inventory, procurement, finance, and compliance.
2
Automated lease & payments
Auto-generated audit logs, approval workflows, and digital traceability for GMP and regulatory standards.
3
Financial and Accounting Integration
Real-time visibility into production costs, material usage, expenses, and profitability.
4
Quality Control & QA Automation
Standardized QC workflows, inspection checkpoints, and automated non-conformance tracking.
5
Batch & Inventory Tracking Dashboard
Centralized monitoring of raw materials, batch numbers, expiry dates, and finished product movement.
6
Scalable Cloud Architecture
Supports multi-site production, R&D facilities, global distribution, and growing pharma operations.
The Results
The integrated Odoo ERP system improved regulatory compliance, enhanced production coordination, and increased operational transparency across Morocco’s pharma operations. Automation reduced manual errors, while real-time dashboards enabled faster decision-making and complete batch-level traceability. Manufacturers achieved smoother production cycles, reduced waste, and stronger control across all stages of drug manufacturing and distribution. The unified environment also strengthened collaboration between QA, production, and supply chain teams, ensuring faster batch release and consistent product quality. As a result, pharma companies gained the reliability and scalability needed to meet both local and international regulatory standards.
Contact Us
Share a few details about your project, and we’ll get back to you soon.
Let's Talk About Your Project
- Free Consultation
- 24/7 Experts Support
- On-Time Delivery
- sales@sdlccorp.com
- +1(510-630-6507)
